Radium-223 (Alpharadin) A Novel Targeted Alpha-Emitter for Bone-Metastatic Castrate-Resistant Prostate Cancer
By Oliver Sartor, MD
If a prostate cancer patient develops metastases in the course of his disease, bone is the most common site. Approximately 90% of these metastatic patients will have at least one bone lesion.
This has led to the concept that a bone-targeted therapy may prolong survival. Although this concept is attractive and logical, actual proof has only recently been demonstrated.
Bone-targeted therapies such as zoledronic acid (Zometa), denosumab (XGEVA), samarium-153 EDTMP (Quadramet) and strontium-89 (Metastron) have been tested in large phase III randomized trials, but none of these agents have been proven to prolong overall survival, unlike agents such as docetaxel, sipuleucel-T, cabazitaxel and abiraterone (1-4).
Radium-223 (or Alpharadin) is a new targeted alpha-emitting agent which has shown a prolongation of survival in castrate-resistant prostate cancer patients with bone-metastatic disease (5).
To digress for a moment, patients that have failed initial hormonal manipulations such as Lupron, Eligard, Zoladex, or Trelstar, or surgical removal of the testicles, are referred to as having “castrate-resistant” prostate cancer, or CRPC. This state was once referred to as being “hormone-refractory” but now we know that secondary hormonal manipulations can have benefits, so castrate-resistant is the current term in vogue.
This brief article is designed to help patients understand radium-223, which is currently available for expanded access trials in select locations (please see page 26). Submission to the FDA for radium-223 regulatory approval is ongoing at this time. The concept of a targeted alpha-emitting therapy will likely evolve in the years to come. In my opinion, this is a first step - not a last.
BASIC CONCEPTS IN RADIATION
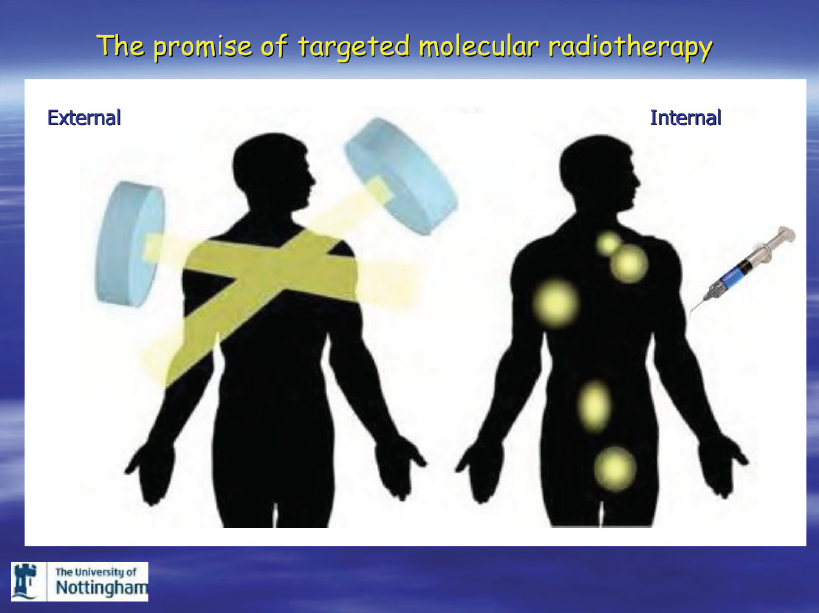
Radiation is administered in three primary forms: external beam radiation therapy (EBRT), brachytherapy (radioactive seeds) and injectable radiopharmaceuticals.
Image courtesy DocStoc.com
External beam radiation is commonly used to treat localized or regionally advanced prostate cancer with a curative intent.
It is also commonly used to treat bone metastases, primarily to provide pain relief.
Administration of external beam involves a machine which delivers and focuses a radiation beam to the desired area. Both photons and protons are used in the external beam setting, though protons are rarely used for treatment of metastatic lesions.
External beam radiation has both advantages and limitations. The beam will go where it is aimed, but cancers outside of the beam are not affected. Because physicians are imperfect in determining the location of cancers, sometimes radiation fails to control the disease. Though radiation will reliably kill cancer in the “fields” of administration, normal tissues in the radiation path are also damaged, and side effects are related to normal tissue damage.
Internal (injectable) forms of radiation have been used to treat patients with bone metastases for over 50 years. Two forms of injectable radiation, strontium-89 and samarium-153-EDTMP, have been FDA-approved for use on bone-metastatic castrate-resistant prostate cancer patients. These compounds can relieve pain, but do not prolong survival. Both of these compounds are radioactive and emit radiation in the form of an electron (also called a beta-particle). The strontium-89 has a long half-life of slightly over 50 days, while samarium-153 has a half-life of only 1.9 days. These compounds bind to the “stroma” surrounding bone metastatic lesions, and then irradiate both the tissue adjacent to the tumor and the tumor itself. Some bone marrow is usually radiated as well. This can lead to lower blood counts in some men.
Brachytherapy, or radioactive seed therapy, is another form of localized radiation. In this case, seeds are carefully placed in the prostate and a high dose is delivered to the tissues adjacent to the seeds. Radioactivity will kill tumors adjacent to the seeds but not affect tumors that are several centimeters away.
CONVENTIONAL INJECTABLE FORMS OF RADIATION THERAPY
How do injectables localize to areas of bone metastases? Prostate cancer bone metastases have a peculiar trait. They are typically “osteoblastic” or “bone-forming” (called blastic or sclerotic). This means that on X-rays or CT scans, the metastatic lesions show up as an area of hyper-dense bone. When viewed on a microscopic level, the region surrounding the cancer cells (the stroma) contains increased new bone formation and increased amounts of calcium and phosphate.
Strontium-89 is a part of the periodic table termed the alkali earth metals. This group of compounds behaves similarly to calcium in biologic systems. Samarium-153 does not bind to the stroma of osteoblastic metastases, but when bound to a compound called EDTMP, it binds avidly to highly calcified regions of bone. Both strontium-89 and samarium-153 EDTMP are beta particle emitters, and both are FDA-approved on the basis of randomized phase III studies that demonstrated an improvement in pain. Both of these agents represent important steps forward in the evolution of radiation therapy.
WHAT ARE BETA AND ALPHA PARTICLES?
Beta particles are electrons that are emitted from an unstable nucleus. Alpha particles are also emitted from unstable nuclei, but are about 7,000 times larger (see Table 1). They are comprised of two protons and two neutrons, and are equivalent to a helium nucleus. The beta particles travel at nearly the speed of light, and alpha particles travel at about 10 percent of the speed of light. Both particles interact with surrounding molecules and can cause cell death via DNA breakage. DNA contains the genetic code, and replication of DNA is essential for cellular proliferation.
Cells with damaged DNA often die in the process of cell division, as the DNA replication cannot successfully occur. Beta particles are more likely to cause single-strand DNA breaks (the DNA is a double helix with two strands). Alpha particles are more likely to cause highly lethal double strand breaks (6).
Calculations indicate that only 1-10 alpha particle “hits” per cell can result in cell death compared to beta particles, which require literally thousands of hits. Because of the large size of alpha particles, the distance traveled is very small. Small particles are less likely to interact with other molecules. Most alpha particles travel less than 100 microns (one micron is equal to 1000th of a centimeter). Beta particles travel various distances, but typical beta particles from strontium-89 or samarium-153 travel about 0.2-2.0 centimeters. Various beta emitters have been used in medicine, but alpha particles are new to medicine, and Radium-223 is the first alpha-emitter to be tested in a large clinical trial.
Table 1: Comparison of Alpha and Beta Particles
RADIUM-223: BASIC CONCEPTS
Radium-223 is an alkali earth metal similar to strontium-89. As such, it is calcium-mimetic, and binds to the stroma in regions of osteoblastic metastases. It has a physical half-life of a little over 11 days.
Radium-223 is an alpha-emitter. This ability of alpha particles to travel only a short distance from the site of origin, combined with their highly destructive cellular effects, results in a maximum amount of cellular killing in regions adjacent to their deposition and a minimal amount of damage to other tissues. Thus, the side effects of alpha particle radiation are minimal because the particle has minimal effects on normal tissues. Only the tumor cells in close proximity to the osteoblastic metastatic lesion are radiated. Thus, radium-223 is a highly targeted alpha-emitter that localizes specifically to the osteoblastic lesions typical of metastatic prostate cancer.
CLINICAL DATA WITH RAD-223
Though alpha-particle radiation targeted to osteoblastic metastatic lesions has theoretical advantages, data from clinical studies always constitute the proof necessary to ensure that a concept is both safe and effective.
The first human studies with radium-223 in patients with prostate cancer established the effective targeting of osteoblastic bone metastases. Once targeting was established, dose ranging studies were performed to define safety. During these studies, both safety and pain relief were noted. A small randomized trial of 64 patients with bone-metastatic “castrate-resistant” prostate cancer was performed. All men received radiation to bone and then were randomized to four doses of radium-223 or placebo. Interestingly, in this small trial, the radium-223 treated patients lived longer (7)(see Figure 1).
Figure 1: Overall survival in the phase III study of radium-223 and placebo (7)
Based on the survival advantage in the small randomized trial, a larger randomized trial (the ALSYMPCA trial) with over 900 patients was designed to assess effects on survival. This trial enrolled symptomatic patients with bone-metastatic CRPC who lacked liver, brain or lung involvement. No lymph nodes larger than three centimeters were allowed. This was done to minimize the enrollment of patients with disease outside of the bone, a population unlikely to benefit from radium-223. Patients had to have been treated with docetaxel or be considered by their physician to be “unfit” for docetaxel, or to refuse docetaxel. Docetaxel is the standard chemotherapy for metastatic CRPC that has been approved by the FDA since 2004. Blood counts and various metabolic parameters, including liver and kidney function, had to be either normal or close to normal. Patients were randomized to receive six intravenous doses (four weeks apart) of Rad-223 plus best supportive care, or placebo injections plus best supportive care. Best supportive care basically included a variety of secondary hormonal manipulations, but no chemotherapy was allowed and no other intravenous radio-isotopes were allowed. All patients continued on their Lupron, Eligard, Zoladex and Trelstar-type drugs so as to avoid a rise in testosterone (which is associated with disease progression in CRPC).
More than 900 patients were enrolled in the ALSYMPCA trial, but an early “interim” analysis was performed to ensure that safety and ethical issues were appropriate for trial continuation.
Surprisingly, this interim analysis (performed after 314 deaths) demonstrated a strong and positive survival advantage for those treated with the radium-223. In the interim analysis, patients in the placebo group lived a median of 11.2 months, while the patients in the radium-223 group lived a median of 14.0 months (5). Please note that this trial, along with every other trial has considerable heterogeneity around the median. This compares favorably with other trials performed predominantly in patients previously treated with docetaxel (3,4). The probability of this result being due to chance was less than 2 in 1,000. A final analysis has recently been reported with more follow-up: The median survivals improved with the placebo group living a median of 11.3 months and the radium-223 group treated a median of 14.9 months (8).
The positive survival results led to the trial being stopped at the interim analysis. It was considered unethical to continue to treat people with placebo, given the extent of the survival advantage with radium-223. In addition to improved survival, the overall safety profile of the radium-223 was excellent. The side effects were unusual, with less adverse events being reported in the placebo arm. Bone marrow suppression, though a potential significant toxicity, was rare (less than 5% of patients).
SUMMARY
Radium-223 will be reviewed by the FDA sometime in 2012. If the FDA approves the drug, it should be available shortly thereafter in selected centers. The current “expanded access trial” has been approved by the FDA in accordance with strict guidelines similar to that of the large phase III trial. This expanded access trial is now open in select cities (please see page 26 for current availability). Exact dates depend on the approval by local Institutional Review Boards and other regulatory agencies.
It is clear that radium-223 will be used in combination with other agents for patients with CRPC. Combining it with various newer hormonal agents seems logical. Combinations with docetaxel (Taxotere) will need to proceed in clinical trials first, as there are unknown safety issues that could be encountered. There is a good rationale for combining radiation with immune modulators, and these trials will begin in the not too distant future. Most other cancers are treated with multiple agents in combination, and prostate cancer will follow this paradigm in the near future.
Radium-223 may be the first step in new therapeutic approach to prostate cancer, and other cancers as well. Alpha particles will prolong survival when properly targeted, providing an important proof of concept. The next challenge is targeting these particles to regions of cancer outside the bone. Various antibodies and other targeting agents may be conjugated together with alpha-emitters to form new targeted forms of radiation therapy.
References
1. Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus
prednisone for advanced prostate cancer. N Engl J Med 2004; 351:1502-12.
2. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate
Cancer. New England Journal of Medicine 2010;363:411-22.
3. de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for
metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open label trial. The Lancet 2010; 376:1147-54.
4. de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011;364:1995-2005.
5. Parker C, Heinrich D, O’Sullivan JM, et al. Overall survival benefit of radium-223 chloride (Alpharadin) in the treatment of patients with symptomatic bone metastases in castration-resistant prostate cancer (CRPC): a phase III randomized trial (ALSYMPCA). Eur J Cancer 47:Suppl 2, Sept 2011, p3.
6. Ritter MA, Cleaver JE, Tobias CA. High-LET radiations induce a large proportion of non-rejoining DNA breaks. Nature 1977; 14;266:653-5.
7. Nilsson S, Franzén L, Parker C, et al. Bone-targeted radium-223 in symptomatic, hormone refractory prostate cancer: A randomized, placebo-controlled, phase 2 study. Lancet Oncol 2007; 8:587-94
8. http://www.algeta.com Feb 10, 2012 press release
Originally posted in PCRI Insights Magazine, May 2012
Dr. Oliver Sartor is the only medical oncologist in Louisiana specializing in the treatment of prostate cancer patients. Dr. Sartor, the Laborde Professor of Cancer Research in the Medicine and Urology Departments of Tulane School of Medicine, helps to complete the world-class prostate cancer team at the Tulane Cancer Center. Together, Lee, Sartor and Thomas are overseeing the prostate cancer program that offers state-of-the-art clinical and surgical care. Before joining Tulane’s faculty, Sartor was an associate professor in the Lank Genitourinary Oncology Center at Harvard’s Dana-Farber Cancer Institute in Boston. He is also chair-elect of the Department of Defense Prostate Cancer Integration Panel, which directs over $70 million in federal funding for prostate cancer research.